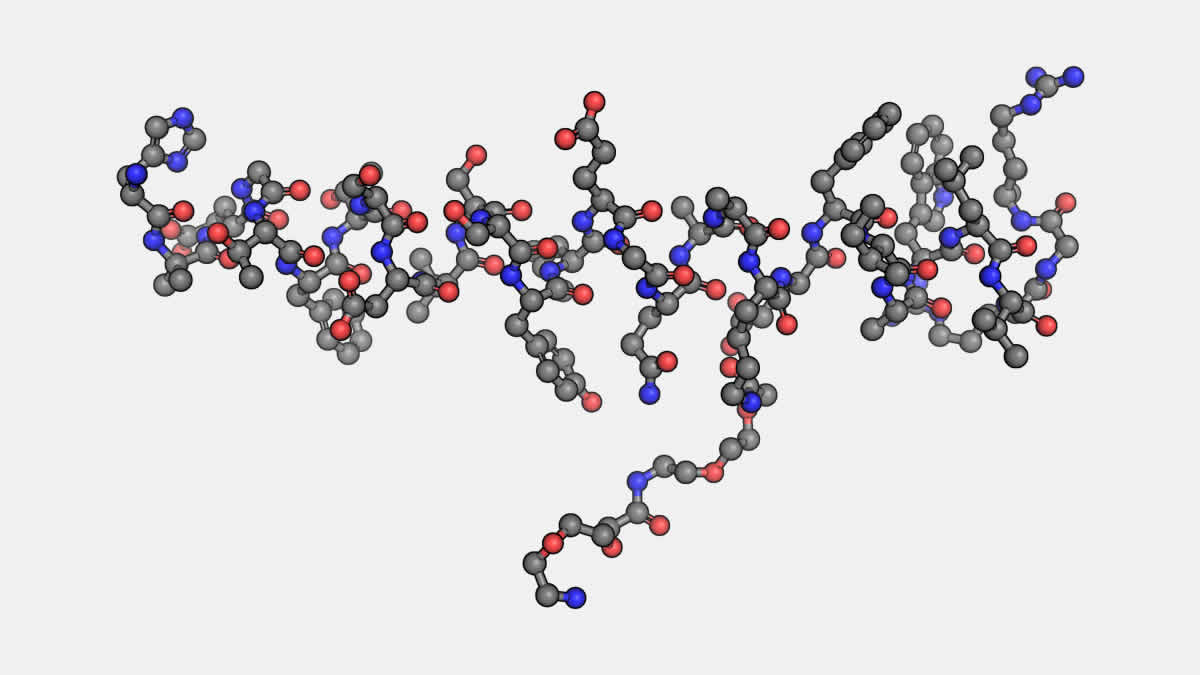
The peptide Semaglutide, a synthetic analog of the glucagon-like peptide-1 (GLP-1), has garnered significant attention in molecular and cellular research. As an agonist of the GLP-1 receptor, Semaglutide is believed to interact with this receptor in a manner that may unlock diverse physiological and biochemical mechanisms. By mimicking the endogenous GLP-1 peptide, Semaglutide might offer a compelling platform for the study of complex signaling pathways. Its potential implications for understanding cellular communication, metabolic modulation, and neuroendocrine integration make it a subject of immense scientific curiosity.
GLP-1 Receptor: A Molecular Conductor in Cellular Signaling
The GLP-1 receptor (GLP-1R), a G-protein-coupled receptor (GPCR), plays a central role in cellular signaling. It is widely distributed across numerous tissues, including pancreatic beta cells, neurons, and cardiovascular structures. GLP-1R activation initiates intracellular signaling cascades involving cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), as well as alternative pathways such as extracellular signal-regulated kinases (ERK).
Studies suggest that Semaglutide, as a GLP-1R agonist, may amplify these signaling dynamics, offering a window into the modulation of transcriptional and translational processes. Investigations have proposed that Semaglutide’s interaction with GLP-1R might extend beyond metabolic regulation to impact cell proliferation, apoptosis, and intercellular communication. Such properties suggest avenues for research into tissue repair, organ homeostasis, and bioenergetics.
Metabolic Insights and Energy Research
The hypothesized role of Semaglutide in regulating glucose metabolism is a cornerstone of its significance in research. By engaging GLP-1R, Semaglutide appears to influence enzymatic pathways that modulate glycogen synthesis, gluconeogenesis, and cellular glucose uptake. This interaction might be pivotal in understanding how energy is distributed and utilized across different systems.
Furthermore, studies suggest that Semaglutide may provide insights into mitochondrial function and oxidative stress responses. The peptide’s possible impact on cellular energy sensors, such as AMP-activated protein kinase (AMPK), and its potential to modulate reactive oxygen species (ROS) levels underscore its relevance in bioenergetic studies. This opens the door to exploring adaptation to metabolic challenges, such as nutrient deprivation or increased energy demands.
Neuroendocrine Dynamics and Cognitive Implications
The neuroendocrine role of GLP-1 and its analogs, including Semaglutide, has been theorized to extend into realms of cognitive function and neural plasticity. GLP-1R expression in the central nervous system (CNS), particularly in the hypothalamus and hippocampus, suggests a regulatory axis linking metabolic states with neural activity.
Research indicates that Semaglutide’s interaction with neural GLP-1Rs may provide insights into mechanisms underlying synaptic signaling, neuroprotection, and memory formation. By influencing calcium influx and synaptic vesicle release, Semaglutide is hypothesized to impact neurotransmitter release and receptor sensitivity. Such hypotheses may drive future investigations into neurodegenerative conditions, neuroinflammation, and learning processes.
Cardiovascular and Vascular Research
The cardiovascular implications of GLP-1 receptor activation have been a growing focus in molecular biology. Semaglutide’s potential to interact with vascular endothelial cells and smooth muscular tissue highlights its relevance in studying circulatory dynamics. It has been proposed that Semaglutide may influence nitric oxide (NO) synthesis and endothelial barrier integrity, which are critical in maintaining vascular homeostasis.
Moreover, Semaglutide’s potential impacts on lipid metabolism and inflammatory markers position it as a candidate for research into atherogenesis and vascular remodeling. By exploring these pathways, researchers might gain deeper insights into the mechanisms that govern circulatory resilience and tissue perfusion.
Immunological Interactions and Inflammatory Research
GLP-1 receptor activity has been hypothesized to modulate immune responses. Semaglutide, through its receptor interactions, is theorized to influence macrophage polarization, cytokine release, and lymphocyte activity. These interactions might shed light on the crosstalk between metabolic states and immune function, a field of particular relevance in chronic inflammatory conditions.
Research into Semaglutide’s role in immune cell metabolism, often referred to as immunometabolism, may reveal how energy pathways intersect with immune activation. The peptide’s potential to modulate inflammatory mediators may offer a framework for understanding systemic inflammation and tissue-specific immune responses.
Implications for Tissue and Cellular Research
The interplay between GLP-1R signaling and cellular repair mechanisms has emerged as an intriguing area of exploration. Semaglutide’s influence on pathways like PKA and ERK suggests it might support cellular survival and regeneration under stress conditions. This property might have implications for understanding wound healing, fibrosis, and organ-specific recovery processes.
In tissue engineering and regenerative science, Semaglutide’s potential impacts on stem cell differentiation and angiogenesis are particularly compelling. Its interaction with growth factor pathways may provide a scaffold for designing interventions aimed at restoring tissue architecture and function.
Prospective Domains for Semaglutide Research
The versatile properties of Semaglutide make it a promising candidate for investigation across several scientific domains:
- Endocrine Signaling and Metabolic Integration: Investigations purport that semaglutide might contribute to a broader understanding of energy homeostasis by elucidating how GLP-1R agonism influences hormonal and metabolic networks.
- Neurological Studies: The peptide’s potential role in neural communication and plasticity may advance research into neuroendocrine regulation and CNS resilience.
- Cardiovascular Research: Investigating Semaglutide’s interactions with vascular and myocardial tissues may provide novel insights into circulatory adaptation and cellular resilience.
- Cellular Stress Responses: By examining Semaglutide’s impact on oxidative stress and mitochondrial function, researchers might uncover new dimensions of cellular defense mechanisms.
- Inflammatory Pathways: The peptide’s hypothesized role in immune regulation positions it as a subject for studying the interface between metabolism and immunity.
- Regenerative Biology: Semaglutide’s potential to influence cellular repair and tissue regeneration underscores its relevance in translational research aimed at developing bioengineered solutions.
Conclusion
Findings imply that Semaglutide, through its interaction with the GLP-1 receptor, represents a promising avenue for expanding scientific data in diverse fields. Its potential to modulate signaling pathways, cellular metabolism, and intersystem communication positions it as a valuable tool for dissecting complex biological processes. While much remains to be uncovered, the peptide’s hypothesized impacts across metabolic, neurological, cardiovascular, and immunological domains underscore its potential as a cornerstone of future scientific inquiries.
By continuing to explore the multifaceted properties of Semaglutide, researchers may unlock pathways that advance both fundamental understanding and applied science, paving the way for innovative approaches to addressing key biological questions. For more educational resources visit this Semaglutide study.
References
[i] Campbell, J. E., & Drucker, D. J. (2013). Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabolism, 17(6), 819–837. https://doi.org/10.1016/j.cmet.2013.04.008
[ii] Holst, J. J., & Rosenkilde, M. M. (2020). Glucagon and GLP-1 receptor signaling: Impact on the pancreatic islets and beyond. Trends in Endocrinology & Metabolism, 31(6), 451–463. https://doi.org/10.1016/j.tem.2020.02.003
[iii] Nauck, M. A., & Meier, J. J. (2018). Incretin hormones: Their role in health and disease. Diabetes, Obesity and Metabolism, 20(S1), 5–21. https://doi.org/10.1111/dom.13129
[iv] Hunter, K., & Hölscher, C. (2012). The neuroprotective effects of GLP-1 receptor agonists in Alzheimer’s and Parkinson’s disease: An update. Progress in Neurobiology, 96(2), 216–233. https://doi.org/10.1016/j.pneurobio.2012.01.005
[v] Yabe, D., & Seino, Y. (2011). Two incretin hormones GLP-1 and GIP: Comparison of their actions in insulin secretion and beta-cell preservation. Progress in Biophysics and Molecular Biology, 107(2), 248–256. https://doi.org/10.1016/j.pbiomolbio.2011.07.010